Abstract
Introduction. Adoptive cell therapy (ACT) with virus specific T cells (VST) has been shown to be safe and effective in controlling viraemia in recipients of haemopoietic stem cell transplant (HSCT). Long term engraftment has been demonstrated in some studies but the fate of the transferred cells is not well understood. We hypothesised that the clinical effect of ACT is mediated by interaction of transferred cells with a wide range of innate and adaptive immune cells present in the recipient at the time of ACT treatment. In order to better understand the interaction of stem cell donor-derived and adoptively transferred third-party cells, we sought to apply immune profiling by mass cytometry.
Methods. Longitudinal samples from healthy individuals and collected pre- and post-donor derived and third party ACT in HSCT recipients were assessed with a 37 marker panel to identify canonical immune subsets. Cryopreserved peripheral blood mononuclear cells were thawed in batches and stained with metal-labelled antibodies and markers for DNA content and viability. Samples were acquired on the Helios upgraded CyTOF2 mass cytometer. Following sample QC and normalisation of files, viable cells were manually gated to 75 canonical populations with cell subsets enumerated and represented graphically for each sample using visualised t-stochastic neighbour embedding (ViSNE). Cell subsets were expressed as percent of total cells as well as absolute cell number (x109/L) using lymphocyte and monocyte counts from an automated whole blood analyser. This approach was taken to incorporate the large numerical changes that occur in the early months post-HSCT. Mass cytometry data was analysed with unsupervised and supervised clustering algorithms to identify immune signatures. Significance of microarray (SAM) was used to identify the cell subset changes that contributed to the differences in clusters. t-stochastic neighbour embedding (tSNE) and principal component analysis (PCA) were used to correlate immune signatures with clinical variables including patient, transplant and donor baseline characteristics and post-transplant outcomes (including CMV reactivation and disease, acute GVHD, relapse and death).
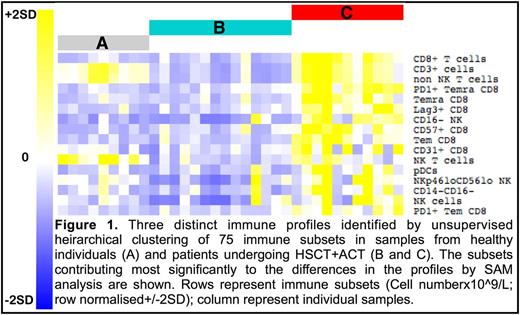
Results. A total of 104 samples from 9 healthy donors and 27 HSCT recipients who received ACT on phase I and II clinical trials were available for analysis. ViSNE revealed highly detailed relationships of the 75 cell subsets within individuals longitudinally and between patients. Unsupervised clustering on all patients and time points and the healthy individuals revealed three distinct immunological signatures (Figure 1). Healthy individuals (signature A) were clearly distinguished from all patients and no patient developed a normal immune profile over the timeframe of the study (up to 6 months post HSCT). In a subset of samples from HSCT recipients a distinct immune phenotype was seen (signature C), characterised by increased CD8+ subsets (CD8em, CD8emra (both of these with and without PD1), CD8+CD57+, CD8+CD31+), NK cell subsets (CD16-, NKp46loCD56lo), NKT cells and plasmacytoid dendritic cells. Of the clinical parameters assessed, signature C was most strongly correlated with CMV reactivation. Signature B showed lower cell numbers overall and included patients with acute GVHD treated with corticosteroids and patients in whom CMV reactivation progressed to CMV disease.
Conclusion. Mass cytometry and sophisticated analysis reveal an unprecedented level of detail about the relationships of immune subsets to one another at single time points and their changes over time. This approach identifies distinct global immune signatures involving changes to multiple immune subsets in patients undergoing HSCT and ACT. We have identified an immune signature associated with CMV reactivation that indicates a broader effect of CMV reactivation on immune recovery than previously recognised. As this study was not randomised and did not include a control group who were not treated with ACT, further investigation is required to define the contribution of ACT to the observed immunological changes. This global approach to immunological assessment has the potential to improve understanding of the dynamics of immunological recovery post HSCT.
Gottlieb: Indee: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal